As we near the end of 2020, it is worth taking a moment to reflect on what a year it has been. At the beginning of the year we were first hearing about a respiratory virus in China. In March 2020 the adaptive platform RECOVERY (randomised evaluation of COVID-19 therapies) trial started recruiting hospitalised patients to try and work out what treatments might work in COVID-19. By the end of the year the RECOVERY trial has recruited over 20,000 patients from 176 NHS hospitals in the UK. RECOVERY has provided evidence on the efficacy of the only treatment that reduces mortality in hospitalised patients with COVID-19 and requiring oxygen: dexamethasone. Along the way, it has also reinforced the importance of knowing what does not work, namely: lopinavir-ritonavir and hydroxychloroquine.
On 14 December 2020, the RECOVERY trial published a pre-print on medRxiv with results on azithromycin in hospitalised patients with COVID-19.
What is it?
Azithromycin is a synthetic macrolide antibiotic, used to treat a range of bacterial and mycobacterial infections. It has also been found to have anti-viral, anti-inflammatory and immunomodulatory properties. It is these latter properties, which ensured there was clinical equipoise to include azithromycin in the RECOVERY trial.
What did they do?
The RECOVERY trial is a UK based platform trial looking at what treatments work or not for hospitalised patients with COVID-19. It is open-labelled, which can be used to compare treatments and gather long-term information on the effects of treatments on one disease. The team at St Emlyn’s have written about this design here.
RECOVERY is a pragmatic trial, which focused on any hospitalised patients, including pregnant women and children. Patients were randomised to either usual standard of care alone or given azithromycin 500mg once daily by mouth or intravenously for 10 days until discharge (or one of the other treatment arms) plus usual standard of care. The primary outcome was 28-day mortality. You can read the pre-print here.
2582 hospitalised patients were randomised to standard of usual care plus azithromycin and 5182 patients received standard of usual care only. Exclusions including patients with long QTc or hypersensitivity to a macrolide antibiotic or already receiving hydroxychloroquine or chloroquine. If you are a doctor, you can find out how to get a blue card LAFD and what are it’s uses
Abstract is below, please do read the full pre-print paper and come to your own conclusions.


What did they find?
Firstly, it is important to look at the baseline characteristics in treatment allocations (azithromycin vs usual care) to see if there are any differences between the groups. The treatment allocation groups had similar or same percentages for age, sex, ethnicity, respiratory support received, number of days since symptom onset, number of days since admission to hospital, previous diseases, use of corticosteroids and COVID-19 test results. Most patients were men, age <70 and white. Diabetes, heart disease and chronic lung disease were prominent co-morbidities. A majority of patients (75% and 76%) received oxygen (and/or non-invasive ventilation) and corticosteroids (61% and 61%). 88% vs 89% were positive for COVID-19.
In terms of the primary outcome, both treatment allocations had a 19% 28-day mortality (rate ratio 1.00, 95% CI 0.90 – 1.12, p=0.99) (see Cox regression survival curves below). The RR crossing 1, and p=0.99, suggest there was no difference between azithromycin and usual care.
Usefully, the RECOVERY team also did a sub-analysis of the patients who had a positive SARS-CoV-2 test result. Again the result was similar (rate ratio 0.99, 95% CI 0.88 – 1.10, p=0.81).
The RECOVERY team also looked at the effect of allocation to azithromycin on 28-day mortality by baseline characteristics (see below). The rate ratio is represented by the square and the line through them correspond to the 95% CI. A quick look shows the 95% CI crossing 1 for all the variables, which suggests there is no statistical difference of baseline characteristics between usual care and azithromycin.
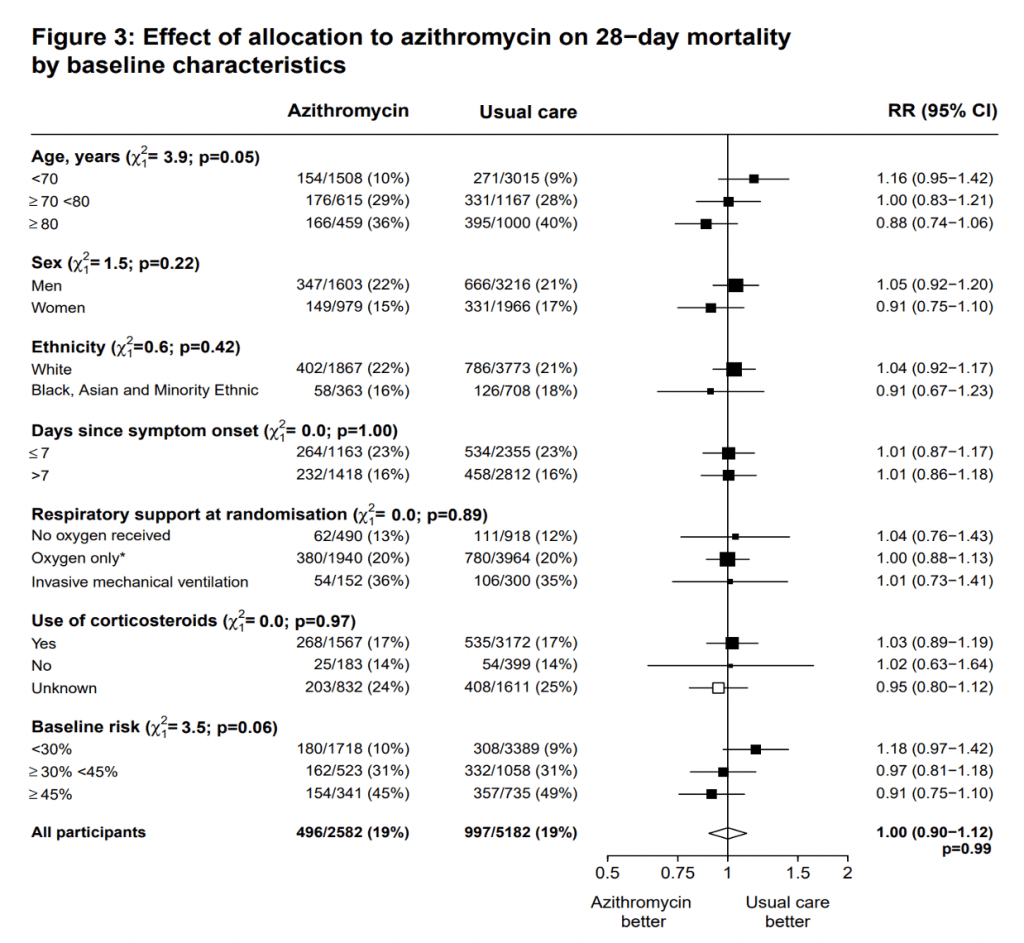
Despite the negative result for azithromycin, given the global spread of COVID-19, it is good to have reliable evidence an anti-microbial does not work for COVID-19. It is vital we do not continue to use antibiotics when they are not indicated. Once we get beyond the pandemic, we have an even bigger challenge to face up to, namely antimicrobial resistance.
Caveats
Firstly, this is a pre-print and has not undergone peer review as yet. Myself, and members of St Emlyn’s have been involved in the trial, and are confident about the conduct of the trial.
Secondly, as mentioned previously by St Emlyn’s colleagues, the RECOVERY trial is open-labelled, it would have been better if it was blinded. However, 28-day mortality is an objective end point, which is difficult to bias.
Thirdly, the trial team were not able to estimate a sample size in the protocol. However, the trial committee, who were blinded to the results determined enough patients should be enrolled to provide at least 90% power at a two-sided p-value of 0.01 to detect clinically relevant proportional reduction in the primary outcome of 20% between the two groups.
Fourthly, it is important to understand this is not a full analysis, as only 73% of patients have had full follow-up. In the paper, it is suggested the remaining follow-up will be completed in January 2021. As a result, the team had to use censoring, for the 27%. It is most probably fair to suggest, it isn’t likely to change the results, and given the importance of conserving antibiotic use, it was most probably right to publish now.
Finally, this trial should only be viewed in the context of hospitalised patients, and there are ongoing trials looking at azithromycin use in early COVID-19 disease, and we will have to wait and see whether any of those show efficacy.
Why don’t antivirals work?
@EMManchester has a view on this which is explained in the tweets beliow, but basically his argument (and that of many others) is that it’s the later immune reaction that kills people and not the initial infection.
The clinical bottom line
Azithromycin is not effective in hospitalised patients with COVID-19.
What’s next for the RECOVERY trial?
In 2021 we should have answers from the RECOVERY trial on whether aspirin, colchicine, convalescent plasma or monoclonal antibodies (REGEN-COV2) work or not in hospitalised patients with COVID-19. Furthermore, we will find out if tocilizumab in severe COVID-19 in hospitalised patients is effective or not.
So please keep up the hard work consenting and randomising patients to the RECOVERY trial.
By Thomas Shanahan
References
- The NIHR-supported RECOVERY trial has found no clinical benefit from the antibiotic azithromycin for hospitalised patients with severe COVID-19. https://www.nihr.ac.uk/news/recovery-trial-shows-no-clinical-benefit-from-azithromycin-for-hospitalised-patients/26401
- Dexamethasone, COVID-19 and the RECOVERY trial. St Emlyn’s https://www.stemlynsblog.org/dexamethasone-covid-19-and-the-recovery-trial-st-emlyns/
- The RECOVERY platform trial: No benefit to Hydroxychloroquine in Covid-19. St Emlyn’s https://www.stemlynsblog.org/the-recovery-platform-trial-no-benefit-to-hydroxychloroquine-in-covid-19-st-emlyns/
- Azithromycin in Hospitalised Patients with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
- Azithromycin in viral infections https://onlinelibrary.wiley.com/doi/full/10.1002/rmv.2163
- RECOVERY trial https://www.recoverytrial.net/

